Laboratory of Katayoon Dehesh at UC Riverside
Research Interests
The Dehesh lab is focused on understanding how organisms perceive and respond to developmental and environmental cues through tightly regulated inter- and intra-cellular communication networks.
The integrity of this communication circuitry enables cellular homeostasis sustained through interorgannellar interactions exquisitely controlled by processes known as anterograde (nucleus-to-organelle) and retrograde (organelle-to-nucleus) signaling. We are specifically interested in communication signals from the metabolic hub, chloroplast, to other cellular organelles.
The overarching objective of our research is to define the nature of retrograde signals, their mode of perception, and the mechanism of signal transduction to optimize adaptive responses. In this quest, we have identified methylerythritol cyclodiphosphate (MEcPP), an intermediate of methylerythritol phosphate (MEP) pathway, as a stress specific retrograde signaling metabolite. MEP is an essential pathway present not only in plants but also in most eubacteria and in apicomplexa that biosynthesize isoprenoids in their non-green plastid-type apicoplast.
We have established that chloroplasts in response to stress adapt the MEP pathway to allow accumulation of MEcPP as a signal to activate a core set of general stress response genes through induction of a transcriptionally centered stress hub. Moreover, MEcPP initiates a stringent transcriptional regulation enabling diversification of secondary metabolites and formation of the associated stress-inducible subcellular structures in the ER. The identification of MEcPP, a precursor of plastidial isoprenoids and a retrograde signaling metabolite initiating these adaptive responses, expands the function of the metabolic hub, plastids, to a stress sensing center providing cues enabling cellular readjustments for high-order adaptive functions.
Current Research Overall Objectives
Defining the Cellular Components of the Plastidial Retrograde Signaling Pathway
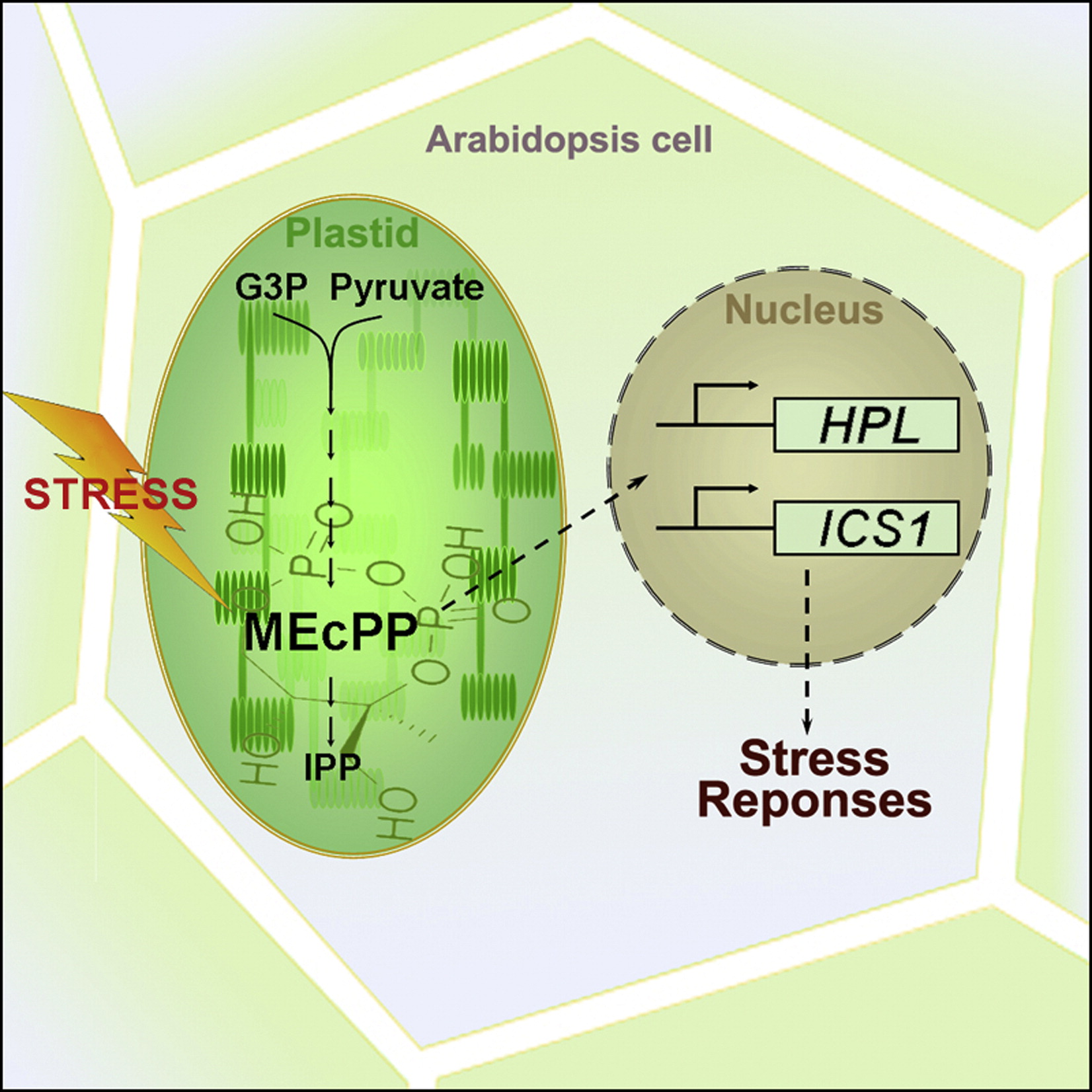
Stress-mediated induction of MEcPP levels functions as a sensor and a communication signal to the nucleus that induces selected stress-responsive genes through alteration of nuclear architecture and functional dynamics.
Unraveling the Molecular Link between Plastids and the ER Unfolded Protein Response
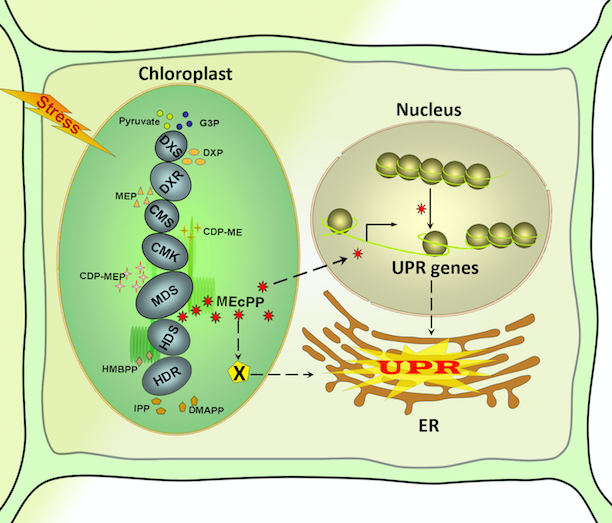
Schematic model depicting alternative routes by which MEcPP potentiates induction of selected unfolded protein response (UPR) genes.
The MEcPP signal may function in the nucleus by first altering chromatin architecture and functional dynamics or by directly modulating a regulator of UPR. Alternatively, the MEcPP signal may, directly or indirectly, potentiate activation of selected UPR genes directly in endoplasmic reticulum (ER).
Unraveling the Molecular Link between Plastids and ER Stress Responses
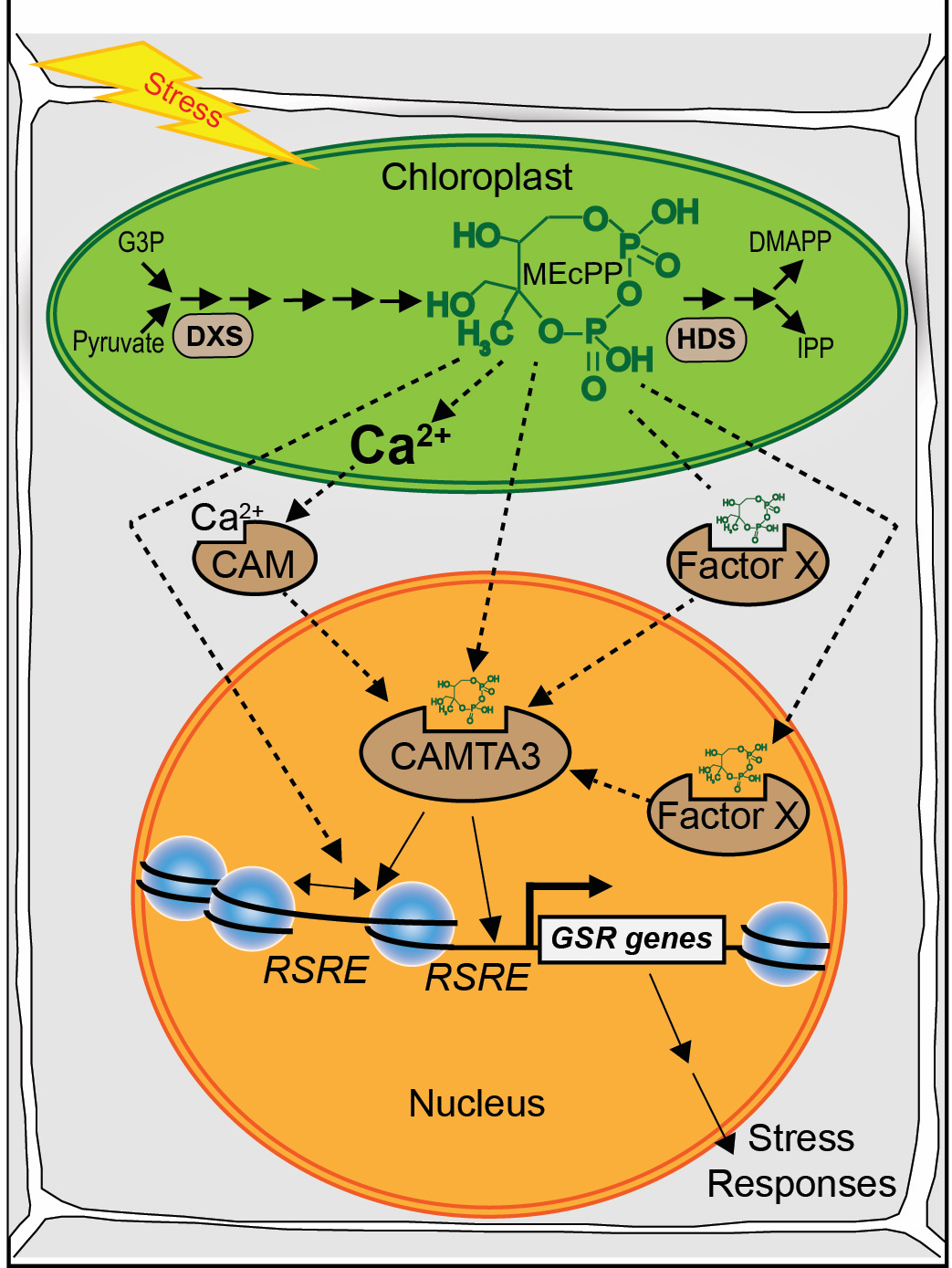
Simplified schematic models of MEcPP mode(s) of action in potentiating the general stress response (GSR).
Possible MEcPP signaling routes are depicted, including stress-mediated alteration in MEcPP levels functioning as a rheostat for the release of Ca2+ for the activation of CAMTA3; MEcPP-mediated alteration of chromatin architecture enabling the accessibility of RSRE for transcriptional regulators; MEcPP potentially functioning as an allosteric modulator of CAMTA3 or its potential coinducer; or a yet unknown transcriptional activator (factor X) binding to and activating RSRE, ultimately triggering the GSR.
Unraveling the Molecular Link between Plastids and ER Stress Responses

The collective parallels between the essential role of the MEP pathway in eubacteria, apicomplexa, and plants expands the importance of our findings well beyond the plantae kingdom. Specifically, our discovery of MEcPP as a novel plastid-to-nucleus, stress-specific retrograde signal offers a unique opportunity to examine how a single primary signal is transduced into adaptive responses critical to the homeostasis of a biological system under unfavorable conditions. Indeed, defining the MEcPP signaling cascade, and how this system transduces the complex array of informational stress signals impinging on plants, may broaden our understanding of adaptive responses in eubacteria and perhaps to malaria.